PDF) Validation of the Sexual Knowledge Picture Instrument as a diagnostic instrument for child sexual abuse: study protocol
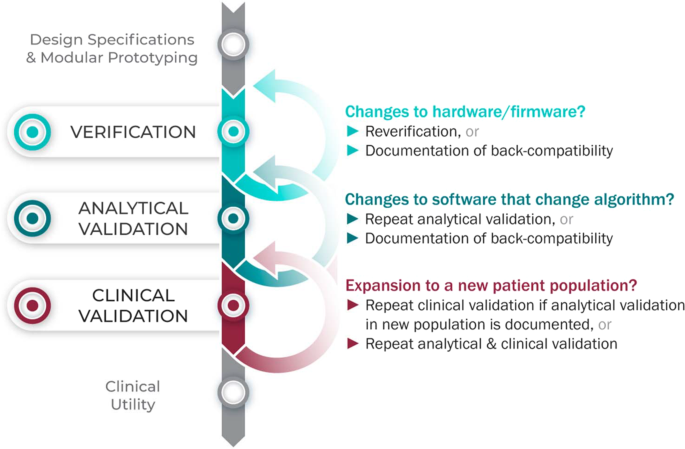
Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs) | npj Digital Medicine
The development and validation of a measurement instrument to investigate determinants of health care utilisation for low back pain in Ethiopia
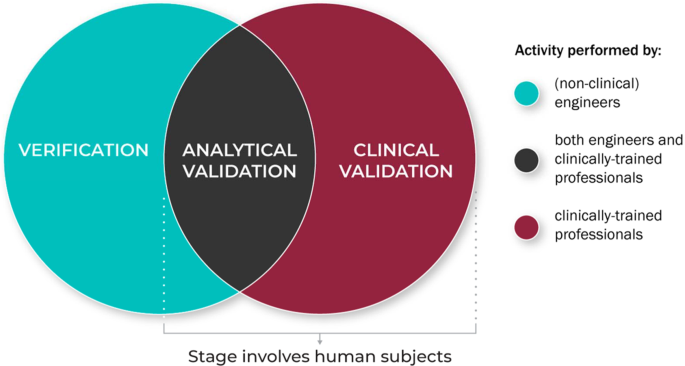
Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs) | npj Digital Medicine

How to Determine the Validity and Reliability of an Instrument | Discovery Center for Evaluation, Research, & Professional Learning

Evaluating the validity, reliability and clinical utility of the Music therapy Sensory Instrument for Cognition, Consciousness and Awareness (MuSICCA): protocol of a validation study

PDF) Cross Cultural Adaptation and Psychometric Validation of Research Instruments: a Methodological Review

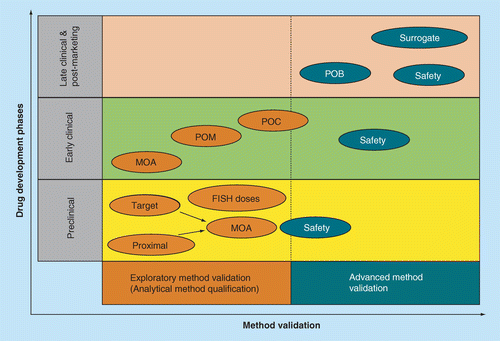
Points to Consider in Quality Control Method Validation and Transfer - BioProcess InternationalBioProcess International
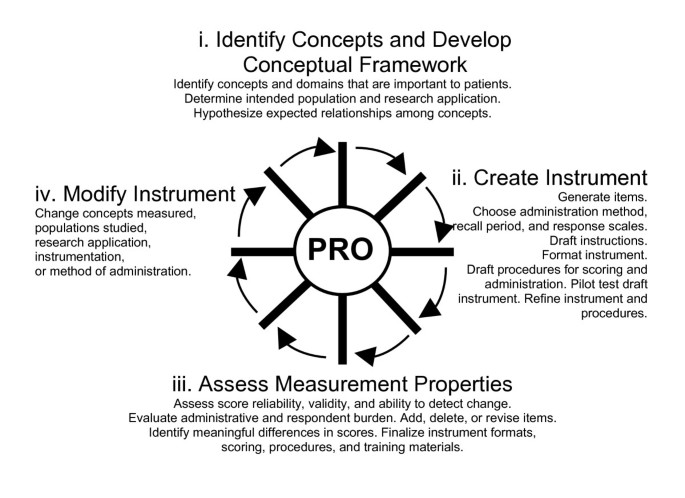
Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance | Health and Quality of Life Outcomes | Full Text